Vitamin C Degradation
Open access peer-reviewed chapter
Vitamin C: An Antioxidant Agent
By Fadime Eryılmaz Pehlivan
Submitted: November 4th 2016 Reviewed: May 9th 2017 Published: August 2nd 2017
DOI: 10.5772/intechopen.69660
Abstract
Vitamin C or ascorbic acid (AsA) is a naturally occurring organic compound with antioxidant properties, found in both animals and plants. It functions as a redox buffer which can reduce, and thereby neutralize, reactive oxygen species. It is a cofactor for enzymes involved in regulating photosynthesis, hormone biosynthesis, and regenerating other antioxidants; which also regulates cell division and growth, is involved in signal transduction, and has roles in several physiological processes, such as immune stimulation, synthesis of collagen, hormones, neurotransmitters, and iron absorption, has also roles in detoxifying the body of heavy metals. Severe deficiency of vitamin C causes scurvy, whereas limited vitamin C intake causes symptoms, such as increased susceptibility to infections, loosening of teeth, dryness of the mouth and eyes, loss of hair, dry itchy skin, fatigue, and insomnia. In contrast, vitamin C can also act as a prooxidant, especially in the presence of transition metals, such as iron and copper, starting different hazardous radical reactions. Vitamin C can both act as a strong, efficient, and cheap antioxidant agent and, at the same time, behave as a radical promoter. Further investigations are needed to illuminate the dual roles of vitamin C
Keywords
- vitamin C
- antioxidant
- ascorbic acid metabolism
- prooxidant
1. Introduction
Vitamin C (l-ascorbic acid) is a water-soluble micronutrient required for multiple biological functions. It is necessary for normal growth and development, and is an essential enzyme cofactor for several enzymes in the post-translational hydroxylation of collagen, biosynthesis of carnitine, conversion of the neurotransmitter dopamine to norepinephrine, peptide amidation, and in tyrosine metabolism. It is also an antioxidant that helps protection against infection and iron absorption. Some animal species have lost the capacity for l-ascorbate synthesis, for that reason, they are dependent upon diet to ensure adequate levels of vitamin C for metabolism and oxidative protection. The high l-ascorbate contents found in plants make them the primary source of vitamin C intake for humans [1–3]. Vitamin C is one of the potent reducing agents and scavenger of free radicals in biological systems, working as a scavenger of oxidizing free radicals and harmful oxygen-derived species, such as hydroxyl radical, hydrogen peroxide (H2O2), and singlet oxygen [3, 4]. Many uses for vitamin C have been proposed, such as its antiscorbutic action, but few have been found to be beneficial in scientific studies [1–4]. In particular, research in stomach cancer, and other cancers, asthma, diabetes, cataracts, or heart disease remains inconclusive. In plants, vitamin C has roles in processes such as growth, programmed cell death, pathogen responses, hormone responses, flowering, and senescence, as well as protection against environmental stresses [1–5]. Vitamin C also plays an important role in abiotic stress tolerance, and considerable interest has focused on it due to its ability to induce a protective effect on plants under stress. It has been supported that vitamin C induced increases in the resistance of plants on heavy metal stress [5]. The role of exogenously applied ascorbic acid (AsA) under heavy metal stress on the photosynthetic pigments, membrane permeability, and mineral uptake of plants is not still clear [5, 6].
Advertisement
2. Biosynthesis and molecular structure of vitamin C
In animals, biosynthesis of vitamin C is included in the glucuronic acid metabolic pathway, which is involved in the metabolism of sugars under normal and disease conditions, and in regulation of physiological functions (Figure 1a). Glucuronic acid metabolic pathway is also an important pathway for detoxification processes [3, 4]. While most animals can convert d-glucose into l-ascorbic acid, humans and other primates, guinea pigs, some fish and birds, and insects are unable to produce ascorbic acid endogenously. The major plant pathway is different from the animal l-ascorbate synthesis pathway that involves 10 enzymatic steps from d-glucose to l-ascorbate via the intermediate formation of GDP-d-mannose and l-galactose [3] (Figure 1a).
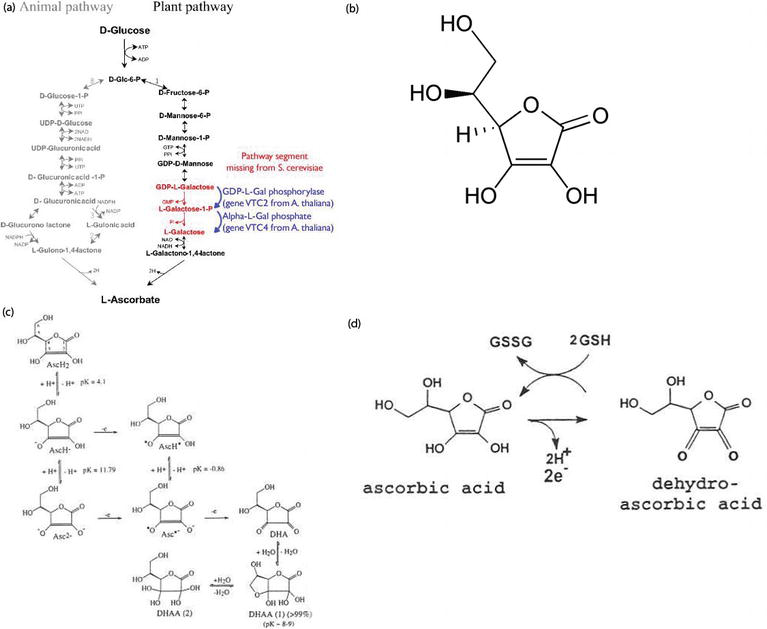
Figure 1.
(a) Diagram for the vitamin C pathway [
Vitamin C (l-ascorbic acid) is a dibasic acid with an enediol group built into a five-membered heterocyclic lactone ring (Figure 1b).
The chemical and physical properties of ascorbic acid are related to its structure [7]. The structure of dehydroascorbic acid, the first oxidation product of ascorbic acid, has been analyzed by X-ray crystallography to be a dimer (Figure 1c).
Electrochemical studies have indicated that ascorbic acid and dehydroascorbic acid form a reversible redox couple (Figure 1d).
Advertisement
3. Redox metabolism and antioxidant properties of vitamin C
Free radicals and oxidants play a dual role as both toxic and beneficial compounds, in metabolic processes and in response to exogenous stimulations. They are produced either from normal metabolic activities or from environmental factors (pollution, cigarette smoke, and radiation). When an overload of free radicals cannot be scavenged, their accumulation in the body generates oxidative stress [3]. Oxidative stress occurs when free radical formation exceeds the ability of protection against them. This process leads in the development of chronic and degenerative illnesses such as cancer, autoimmune disorders, aging, cataract, rheumatoid arthritis, cardiovascular, and neurodegenerative diseases [8–10]. An antioxidant is a molecule that prevents the oxidation of other molecules. Oxidation process is a chemical reaction that produces free radicals, leading to chain reactions that may damage cells. The antioxidant effect of vitamin C has been well documented [8, 9, 11]. Vitamin C is a powerful antioxidant having ability to donate a hydrogen atom and form a relatively stable ascorbyl-free radical. Vitamin E, vitamin C, and β-carotene are known as antioxidant vitamins that are suggested to decrease oxidative damage and lowering the risk of certain chronic diseases. Diseases, such as cardiovascular disorders, are associated with inadequate concentrations of l-ascorbic acid, tocopherol, and β-carotene [8–10] in epidemiological studies. Vitamin C also enhances iron absorption by reducing Fe3+ to Fe2+ from non-heme iron sources [3, 23]. In the presence of redox-active ions (iron, copper), vitamin C acts as a prooxidant, contributing to the formation of hydroxyl radicals, that may lead to lipid, DNA, or protein oxidation [8–10]. There are different mechanisms to alleviate oxidative stress and repair damaged macromolecules. Enzymatic and nonenzymatic antioxidants have important roles in scavenging free radicals and reactive oxygen species (ROS). The antioxidant enzymes, catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GSHpx) and, in plants, ascorbate peroxidase (AA-px) and the nonenzymatic antioxidants, including glutathione (GSH) and ascorbate (ASC), have been shown to be significantly affected by oxidative stress [8, 9]. Antioxidant compounds can prevent the uncontrolled formation of free radicals or inhibit their reaction with biological sites; also, the destruction of most free radicals depends on the oxidation of endogenous antioxidants mainly by scavenging and reducing molecules [8, 9]. Vitamin C is thought to be an important water soluble antioxidant which is reported to neutralize ROS and reduce the oxidative stress [8, 10].
Vitamin C is a potent reducing agent and scavenger of free radicals in biological systems [11]. It is involved in the first line of antioxidant defense, protecting lipid membranes, and proteins from oxidative damage. As a water soluble molecule, vitamin C can work both inside and outside the cells, and can neutralize free radicals and prevent free radical damage. Vitamin C is an excellent source of electrons for free radicals that are seeking out an electron to regain their stability. Vitamin C can donate electrons to free radicals and quench their reactivity [8, 9].
Vitamin C has been shown to be an effective scavenger against oxygen and nitrogen oxide species, such as superoxide radical ion, hydrogen peroxide, the hydroxyl radical, and singlet oxygen. This property of vitamin C has vital processes in protection of cellular components from free radical-induced damage. In addition, vitamin C is effective in regenerating the antioxidant form of vitamin E by reducing tocopheroxyl radicals. This process protects membranes and other compartments of the cell from free radical-induced damage [8, 9] (Figure 2). Ascorbate peroxidase (APX) is an enzyme reducing H2O2 to water by using ascorbate as an electron donor. Monodehydroascorbate is an oxidized ascorbate that is regenerated by monodehydroascorbate reductase (MDAR). Monodehydroascorbate radical rapidly disproportionates into ascorbate and dehydroascorbate. Dehydroascorbate is reduced to ascorbate by dehydroascorbate reductase in the presence of GSH, yielding oxidized glutathione (GSSG). It is reduced by glutathione reductase (GR) using nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) as an electron donor. Dehydroascorbate may be reduced nonenzymatically or catalyzed by proteins with dehydroascorbate reductase (DHAR) activity.
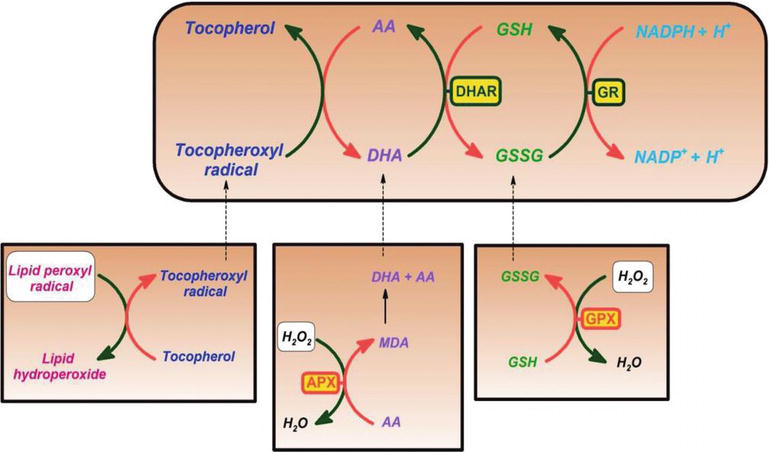
Figure 2.
Ascorbate and redox cycling antioxidants. AA, ascorbate; DHA, dehydroascorbate; DHAR, semidehydroascorbate reductase; GSH, glutathione; GSSG, semi-glutathione reductase; GR, glutathione reductase; APX, ascorbate peroxidase; and GPX, glutathione peroxidase [
Glutathione-ascorbate cycle operates in the cytosol, mitochondria, plastids, and peroxisomes in plants [8, 9]. It is suggested that the glutathione-ascorbate cycle plays a key role for H2O2 detoxification, because of the high concentrations of glutathione, ascorbate, and NADPH in plant cells. Other enzymes, such as ascorbate and glutathione peroxidases, which use thioredoxins or glutaredoxins as reducing substrates, also take roles in the removal of H2O2 in plants [8, 9] (Figure 2).
Vitamin C also forms the semidehydroascorbyl radical, a relatively long-lived radical, in regenerating vitamin E from its radical form, as well as in scavenging radicals. Plant and animal cells contain an NADH-dependent semidehydroascorbate reductase enzyme (EC 1.6.5.4), reducing the radical back to vitamin C by using NADH as a source of reducing agent (Figure 2). Both enzymatically and nonenzymatically, it can irreversibly decompose into diketogluconic acid or it can be converted to ascorbate in a glutathione-dependent reaction [3, 13, 14].
As being a reducing substance and an electron donor, during free radical scavenging, vitamin C donates high-energy electrons to neutralize free radicals, and it is oxidized to dehydroascorbic acid. Dehydroascorbic acid may be converted back into ascorbic acid for reuse or may be metabolized, further releasing more electrons. Although vitamin C is absorbed from the gut via a sodium-dependent vitamin C transporter, most cells transport vitamin C in an oxidized form (dehydroascorbic acid) via glucose transporter 1. Dehydroascorbic acid is reduced to generate ascorbic acid inside the cell, protecting mitochondria from free radical-induced oxidative damage (Figures 2 and 3). Highly reactive free radicals (e.g., RO–, RO2–, OH–,, NO2) are reduced by ascorbate, and the newly generated ascorbyl radical is poorly reactive. Ascorbate can also scavenge nonradical reactive species, derived from peroxynitrite, such as hypochlorous acid, ozone, and nitrating agents. Vitamin C is a monosaccharide oxidation-reduction (redox) catalyst found in both animals and plants. The antioxidant effect of vitamin C is due to its ability to donate electrons from both the second and third carbon. During primate evolution, one of the enzymes needed to make ascorbic acid has been lost by mutation, humans must obtain it from the diet [15]; most animals can synthesize this vitamin in their bodies and do not require it in their diets [16]. Vitamin C is needed in the conversion of the procollagen to collagen by oxidizing proline residues to hydroxyproline. In other cells, it is maintained in its reduced form by reaction with glutathione [17]. As shown in Figures 2 and 3, ascorbic acid is a redox catalyst which can reduce, and thereby neutralize, ROS such as hydrogen peroxide (H2O2) (Figures 2 and 3).
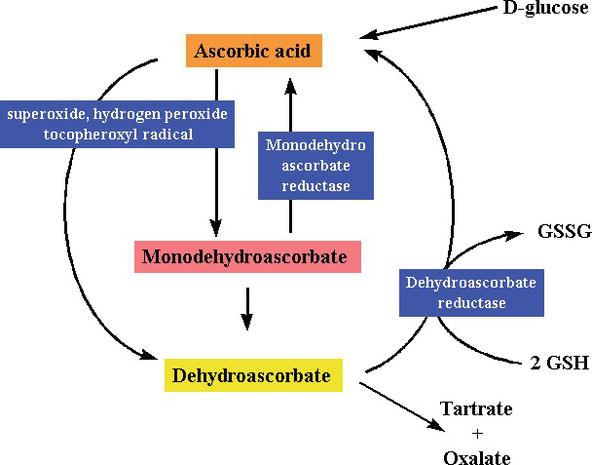
Figure 3.
Synthesis and degradation of
Ascorbic acid has direct antioxidant effects, and also it is a substrate for the redox enzyme ascorbate peroxidase, that is particularly important in stress resistance in plants. Ascorbic acid is present at high levels in all parts of plants, especially in chloroplasts that reach concentrations of 20 mM there [19]. Dehydroascorbate (DHA) and ascorbate free radical (AFR), as an intermediate, the ascorbate free radical (AFR), that are reversible, one-electron oxidations are generated from ascorbate (Figure 4). According to the generally assumed model of enzymatic removal of ROS, SOD catalyzes superoxide anion to H2O2 and oxygen; then H2O2 is reduced into water and molecular oxygen by CAT. CAT turnover number is very high, but its affinity for H2O2 is relatively low, and consequently a certain amount of H2O2 remains in the cell.
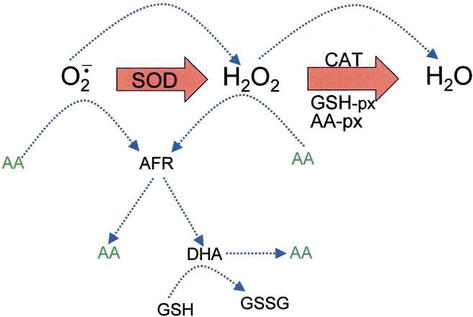
Figure 4.
The role of AA in the detoxification of ROS. Blue dotted lines indicate nonenzymatic reactions.
H2O2 can react with superoxide anion formed in oxidative metabolism generating the highly reactive hydroxyl radical. GSH peroxidases (GSH-px) and AA peroxidases (AA-px) are capable of scavenging H2O2 due to their high affinity for H2O2. The cooperativity of SOD, CAT, and peroxidases ensures low amounts of superoxide anion and H2O2 and limiting the risk of hydroxyl radical formation (Figure 5).

Figure 5.
Foyer-Halliwell-Asada cycle [
Advertisement
4. Role of vitamin C in lipid peroxidation
The chemical and biological properties of l-ascorbic acid suggest that it can act as an antioxidant

Figure 6.
Schematic presentation of ROS-mediated lipid peroxidation chain reaction. Vitamin C serves dual role of a prooxidant and an antioxidant [
LPO• radical can damage the macromolecules (DNA, RNA, and proteins) and can initiate cytotoxic, genotoxic, and inflammatory reactions. Vitamin C converts lipid peroxidation products into unreactive vitamin C-LPO products. This helps to prevent the interaction of macromolecules (DNA, RNA, and proteins) with LPO• radicals. Vitamin C plays role in the regeneration of vitamin E; it donates electron to tocopheryl radical (vitamin-E–O•) and reduces it to tocopherol.
Advertisement
5. Prooxidant effect of vitamin C
The reducing agents, antioxidants, can also act as prooxidants. Vitamin C is also known to act as a prooxidant
E1
E2
Vitamin C is a reducing agent and antioxidant, and reacts with reactive oxygen species, such as the hydroxyl radical. Oxidative modifications of lipids, proteins, and DNA are induced by mixtures of ascorbic acid and copper or iron for decades [24, 25], contributing to oxidative damage formation by reducing ferric Fe3+ to ferrous Fe2+ ions (and Cu2+ to Cu+), which in turn can reduce hydrogen peroxide (H2O2) to hydroxyl radicals. Therefore, vitamin C-mediated Fenton reactions should be controlled in the human body due to efficient iron sequestration by metal-binding proteins such as ferritin and transferrin. It has been suggested that the prooxidant effect may not be relevant
E3
The oxidized forms of ascorbate are relatively unreactive and do not cause cellular damage.
In the presence of free metal ions, excess ascorbate promotes and initiates free radical reactions. This is a potentially dangerous prooxidative compound; thus, vitamin C supplements are not recommended in people with high iron levels [3, 27]. It is provided a mechanism which, vitamin C induces the decomposition of lipid hydroperoxides to genotoxic bifunctional electrophiles
Advertisement
6. Vitamin C in human disease
As an electron donor, vitamin C could be involved in several disease processes. Vitamin C is present in almost all foods of plant origin. The minimal vitamin C requirement for humans is defined as 40–60 mg/day to combat dietary deficiency [28]. However, vitamin C status decreases with both age and smoking, and is associated with chronic diseases such as rheumatoid arthritis and cancer [28]. Vitamin C might be consumed by preventing free radical-induced damage of DNA, which is thought to be an initiating step in cancer formation. The possible use of vitamin C in cancer therapy and prevention has been an area of great interest. Vitamin C supplements, which are able to prevent the formation and/or promote the repair of pre-mutagenic oxidative DNA lesions, are suggested to be of use in cancer prevention. Recently, a report showed that daily supplementation with vitamin C at high doses increased the survival time of terminal cancer patients, suggesting that vitamin C can have important anticancer properties. Indeed, vitamin C is proved to kill or inhibit the growth of many tumor cell lines [28]. Regarding cancer prevention, several epidemiological studies have linked the consumption of a diet rich in fruit and vegetables with lower incidence of many types of cancer [3, 29–34]. The hypothesis supports oxidative processes regulate ascorbate catabolism in humans, although direct proof is currently lacking. Ascorbate plays an important role as a first defense against oxidative stress. Smokers present one example of the relationship between oxidants and ascorbate since they expose themselves to oxidants via inhaled smoke. These oxidants have been demonstrated to induce lipid peroxidation
Advertisement
7. Conclusion
Vitamin C, as an antioxidant agent, has been the object of several investigations. However, vitamin C can also act as a prooxidant, especially in the presence of transition metals such as iron and copper, starting different dangerous radical reactions. As the experimental findings that have not been obtained by studying
© 2017 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
How to cite and reference
chapter statistics
7375 total chapter downloads
18 Crossref citations
More statistics for editors and authors
Login to your personal dashboard for more detailed statistics on your publications.
Access personal reporting
Related Content
This Book
Next chapter
Radioprotective Effect of Vitamin C as an Antioxidant
By Tetsuo Yamamoto and Manabu Kinoshita
Related Book
First chapter
Role of an Atomic-Level-Based Approach for Improving Cancer Therapy
By Santi Tofani
We are IntechOpen, the world's leading publisher of Open Access books. Built by scientists, for scientists. Our readership spans scientists, professors, researchers, librarians, and students, as well as business professionals. We share our knowledge and peer-reveiwed research papers with libraries, scientific and engineering societies, and also work with corporate R&D departments and government entities.
More About Us
Source: https://www.intechopen.com/chapters/56013


0 Komentar